Precise
Precaution versus Sloppy Science
Hartmut Meyer
German NGO Forum on Environment and Development
[Note: Harmut Meyer is a German NGO observer to the biosafety
protocol negotiations. This article was recently published in the Bulletin of Science,
Technology, and Society.]
The Convention on Biological Diversity opens the
possibility to negotiate a legally binding Biosafety Protocol to assess and minimize risks
in the field of transboundary transfer, handling, and use of organisms modified by genetic
engineering. Two principles - the Precautionary Principle and the Principle of Familiarity
- guiding the risk assessment as basis of import decisions on such organisms are
discussed. Developing and European industrialized countries favor the Precautionary
Principle. The US, Australia, Japan, and some others call for the Principle of
Familiarity. These two principles exihibit opposit effects on scientific progress in
general and on scientific methodology of risk assessment in particular. With the example
of risk assessment by the U.S. company Monsanto discussed below, it could be illustrated
that the Principle of Familiarity opens the way for superficial evaluations based on
citing arbitrary references while the Precautionary Principle is an incentive for
developing and applying sound methodology in experimental risk assessment.
Since May 1997, negotiations on a legally binding
Biosafety Protocol to assess and minimize risks in the field of transboundary transfer,
handling and use of living modified organisms (LMOs) are taking place in Montreal, Canada.
LMOs are organisms that are modified by means of genetic engineering. They contain new
combinations of genetic material which could not have been gained by conventional breeding
and that have been transfered between sexually incompatible organisms, thus overcoming
natural barriers of reproduction. The Montreal negotiations focus on the transboundary
transfer of such LMOs, for example, on the international exchange of and trade with
transgenic seeds, grains, animals or bacteria. The negotiations are based on Articles 19.3
and 8(g) of the Convention on Biological Diversity, elaborated during the Earth Summit in
Rio de Janeiro, Brasil, in 1992 and on Decision II/5 of the Second Conference of the
Parties to this Convention in Jakarta, Indonesia, in 1995 (see the appendix; for more
documents, see www.biodiv.org). Until the Fourth Conference of the Parties in 1998, 172
states ratified this Convention. This Conference decided to finish the Biosafety Protocol
in February 1999. In 1998, transgenic crops were allowed to be planted in eight countries
of the world. As the country with the largest acreage of transgenic crops (74%) and as the
main trader of LMOs, the United States cannot become member of the Protocol until they it
has ratified the Convention on Biological Diversity.
Is the Precautionary Principle
Incompatible With Sound Science?
One of the main demands of civil society organizations
during the Montreal negotiations calls for the Precautionary Principle as the basis for
scientific risk assessment and political decision-making under the Biosafety Protocol.
Civil society organizations witnessed that some delegations try to establish contradiction
or even incompatibility between the Precautionary Principle and sound science. It is the
view of civil society organisations that at their best, sound science as a methodological
concept and the Precautionary Principle as guideline for decision-making are mutually
supportive and can operate in perfect harmony.
What does this mean within the context of the Biosafety
Protocol? The Precautionary Principle enhances the search for scientific knowledge by
initiating a scientific risk assessment and thus collecting data and providing scientific
evaluations on ecological, health and socio-economic risks of LMOs on biological diversity
and human health. Sound science serves precaution because it contributes to an early
warning system supporting political efforts to minimize already obvious harm and to
prevent the emergence of new negative impacts.
Different Positions in
the Biosafety Negotiations
However, not all delegates share this concept of mutual
supportiveness between science and precaution. The legal frame work of the US, which is
the leading exporter of LMOs and products therof, stresses the Principle of Familiarity as
basis for decision-making in the context of modern biotechnology (FDA 1992). What task
does this assign to science? Science has to provide risk assessment on the intended novel
traits of a given LMO. However, as far as the unintended changes are concerned, science
has to provide arguments for avoiding further risk assessment and has to support the
judgement that the differences in the composition of genetically engineered and
non-engineered organisms are insignificant. Thus, science is turned into an instrument for
stopping scientific curiosity and hindering the quest for new scientific knowledge. The
promotion of the Precautionary Principle seems to civil society organisations the
appropriate and necessary safeguard to counteract the risks of such science which is based
on and reduced to serving the interests of exporting companies. Precaution is the only
chance for all countries and populations to prevent harm. Moreover, it is the only chance
for poor for mitigating adverse effects of modern biotechnology because they will not be
able to finance remedial actions.
The non-adherance to sound standards of science and the
strange lack of inquisitiveness in the type of science that is not exposed to the stimulus
of the Precautionary Principle will be demonstrated below in the attached evaluation of
Monsanto’s attempt to prove the familiarity of consumers and environment with Roundup
Ready® Cotton.
Case Study: Roundup Ready® Cotton
Roundup Ready® Cotton was engineered by
means of gene technology to resist Monsanto’s herbicide Roundup Ultra®.
In addition to the main product-that is, cotton fiber-residues from processed cotton bolls
are used as animal feed and for human nutrition, notably, cotton protein and cotton oil.
To register herbicide resistant Roundup Ready® Cotton in the United States,
Monsanto had to prove its substantial equivalence to conventional cotton. In 1996, Nida et
al. published an article meant to provide the supporting scientific evidence. One of the
features that were analysed by the authors was the content of gossypol, a toxic terpenoid
constitutent of various parts of the cotton plant.
Nida et al. (1996) showed by their experimental results
that there is a statistically significant difference, and not an equivalence, in the
gossypol content between the seeds of two tested LMOs (n=6, Ms=1.32 and
1.01%) and the parental, non-modified line (n=6, M=1.19%). How did the
authors then proceed to prove that these significant differences are within the range of
familiarity? Did they continue the experimental approach or did they just make use of
available publications? Admittedly, it is cheaper and faster to rely on preexisting
publications. However, choosing an experimental approach is the very way in which science
increases the range and depth of its knowledge. Moreover, this is especially appropriate
for research on unintended effects. Nida et al. quoted, "previously reported
[gossypol] levels for cottonseed grown under various field conditions, 0.39-1.70% (Berardi
& Goldblatt, 1980; Abou-Donia, 1976)"(p. 1871).
The comparision of sets of data generated under
different conditions to prove the equivalence of the analysed biological entities is one
of the most demanding scientific challenges. The experimental approach would imply a
series of comparative analyses, covering LMOs, with their parts and products, and covering
corresponding unmodified organisms, with their parts and products. This should be
preferably performed within the same laboratory applying the same methods. The second
approach compares data taken from publications that were not designed to be stricly
comparable. The nonexperimental approach which Monsanto has chosen (see Figure 1) has to
adhere to the same stringent standards of sound science as the experimental approach (Nida
et al., 1996). Does the article by Nida et al. (1996) do so? One could think so because
their choice of references passed the peer review of the publishing scientific journal.
Monsanto made use of it as proof of substantial equivalence. The Principle of Familiarity
then freed Monsanto from conducting a risk assessment. Are the data comparable and is
their interpretation conclusive?
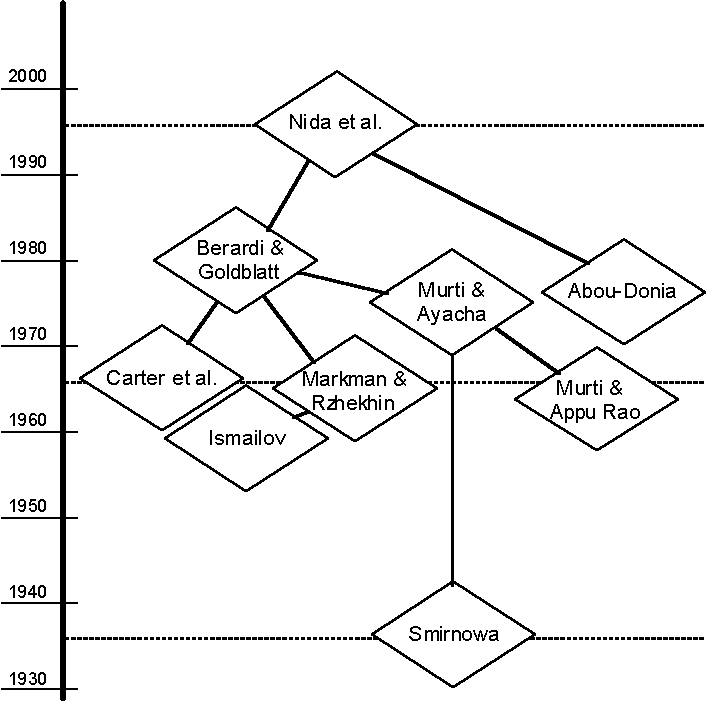
Figure 1. References Cited and Visualization of Their Interrelationship
Analysis of the Publications
Cited by Nida et al. (1996)
Direct References
Abou-Donia (1976) wrote one sentence on the gossypol
content in cottonseed: "Cottonseed usually contains 0.4 to 1.7% gossypol.".
These numbers were cited correctly by Nida et al. (1996). However, Abou-Donia (1976)
neither stated the source of these data, nor the cotton vareties, nor the country and year
of the field trials that provided these data. Therefore, this reference is a very
questionable proof of the gossypol content of cottonseed.
Berardi and Goldblatt (1980) give more details:
Ranges of 0.68-2.36% gossypol in kernels of various
cottonseed varieties grown in India and of 0.33-2.40% gossypol in kernels of seed grown in
the USSR were reported (Markman & Rzhekhin, 1969; Murti & Achaya, 1975). Carter et
al. (1966) reported that gossypol contents of seed from 11 (cultivated and
noncultivated) species of the genus Gossypium varied from 0% for the glandless
varieties of G. hirsutum L. to more than 9% for G. klotzschianum var. davidsoni
(Kellogg). (pp. 192-193)
The reference of Nida et al. (1996) to this set of data
lacks two important features: (a) The numbers are not correctly quoted, and (b) the data
are not comparable, as the different authors are not using the same parts of the cotton
boll for their chemical analysis.
The most central component of the cotton seed is the
kernel which has the gossypol-producing glands. As such, the seed is composed of the
kernel and gossypol-free coating layers (hull and, in North American species, the fibrous
linter layer). Whilst Nida et al. (1996) used delinted seed, Berardi and Goldblatt (1980)
refered to kernels. The concentration of gossypol in kernels is approximately twice as
high as in whole seed.
First Level of Indirect References
Carter et al. (1966) analysed the gossypol content in
kernels of different species of the genus Gossypium, including wild species. This
dated publication provides genuine experimental data. A comparision of a distinct
transgenic cotton line with any other cotton species might be useful for a general
understanding of different levels of gossypol production; yet, to use such a comparision
to establish substantial equivalence through familiarity is seriously doubtful.
Markman and Rzhekhin (1969) reviewed Soviet literature
on the gossypol content of various parts in different cotton varieties and species.
Berardi and Goldblatt (1980) chose one table on kernel data published in that review,
originally compiled by Ismailov in 1959.
Murti and Achaya (1975) reviewed Indian publications on
the composition of various parts in different cotton varieties and species. Data on
gossypol contents in kernels are taken from Carter et al. (1966), Narayana Rao and
Krishnamurthy (1953), Raghavendar Rao (personal communication), and Anonymous (no date).
From this review, Berardi and Goldblatt (1980) have chosen data, originally compiled by
Narayana Rao and Krishnamurthy (1953) and Raghavendar Rao (personal communication). The
publication of Narayana Rao and Krishnamurthy (1953) is not publically available to the
international scientific community; personal communications are in general not appropriate
in a sound risk assessment. In addition to the above citations, Murti and Achaya (1975)
also present data on the gossypol content of whole seeds of four cotton species by citing
the results of Murti and Appu Rao (1963) and Smirnova (1936), respectively. These data are
the only data which may lend themselves to comparison with the LMO data of Nida et al.
(1996), yet they were not used.
Second Level of Indirect References
Ismailov (1959) compiled data on the gossypol content of
cotton kernels from varieties within six cotton species. This publication is not
publically available to the international scientific community. Again as argued above,
kernels and whole seeds are not comparable entities.
Murti and Appu Rao (1963) present data (0.27%-0.82%, as
cited in Murti & Achaya, 1975) that contradict the assumption of substantial
equivalence of Monsanto’s Roundup Ready®-Cotton. The whole seed they used
contained approximately half the amount of the toxin gossypol than did the seeds of
Monsanto’s cotton plants. Again, this publication is not publically available to the
international scientific community.
Smirnova (1936) uses whole seeds (0.15%-1.59%, as cited
in Murti & Achaya, 1975) and has some aspirations to be comparable with Nida et
al.’s (1996) results. Again, this publication is not publically available to the
international scientific community. This data was produced 60 years before Nida et al.,
and methods of analysis have changed. Strict comparability could be better served by more
recent research data.
Conclusion
All documents negotiated in Rio de Janeiro should
introduce the Precautionary Principle as formulated in § 15 of the Rio Declaration into
international legislation. In the field of assessing risks of organisms and their products
altered by genetic engineering, existing European legislation adheres to the Precautionary
Principle while the national legislation in the United States is based on the Principle of
Familiarity. In the Montreal negotiations, the European countries together with the G77,
representing the developing countries, call for the inclusion of the Precautionary
Principle in the Biosafety Protocol. Apart from the advantages that this principle gives
parties to ensure effective health and environment protection, the implementation of the
Precautionary Principle in the Biosafety Protocol will have direct effects on the quality
of science and progress of knowlegde. While the application of the Principle of
Familiarity causes a lack of incentives for new scientific investigations, the application
of the Precautionary Principle will stimulate scientific accuracy and progress in risk
assessment. The above case study on the gossypol content of Roundup Ready®
Cotton is one of several possible examples to illustrates the inediquacy or the Principle
of Familiarity as basis for sound risk assessment. The final round of the Biosafety
Protocol negotiations in Cartagena, Columbia, in February 1999 is the last possibility for
the governments to create a Protocol with innovative and demanding legal and scientific
instruments to ensure optimal protection of human health and biological diversity.
APPENDIX
The Biosafety Protocol Is
Based on International Law
1992 - Convention on Biological Diversity
§ 19.3
The Parties shall consider the need for and the
modalities of a protocol setting out appropriate procedures, including, in particular,
advance informed agreement, in the field of the safe transfer, handling and use of any
living modified organism resulting from modern biotechnology that may have adverse effect
on the conservation and sustainable use of biological diversity.
§ 8 (g)
Each Contracting Party shall, as far as possible and as
appropriate:
. . .
(g) Establish or maintain means to regulate, manage or
control risks associated with the use and release of living modified organisms resulting
from biotechnology which are likely to have adverse environmental impacts that could
affect the conservation and sustainable use of biological diversity, taking also into
account the risk to human health;
. . .
1995 - Jakarta-Mandate II/5:
The Conference of the Parties, . . . Affirming that
international action on biosafety should offer an efficient and effective framework for
the development of international cooperation aimed at ensuring safety in biotechnology
through effective risk assessment and risk management for the transfer, handling, and use
of any LMO resulting from modern biotechnology that may have environmental impacts that
could affect the conservation and sustainable use of biological diversity, taking also
into account the risks to human health, and taking also into account Articles 8(g) and 19,
paragraph 4, of the Convention, . . .
1. Decides to seek solution to the above-mentioned
concerns through a negotiation process to develop, in the field of the safe transfer,
handling and use of living modified organisms, a protocol on biosafety, specifically
focusing on transboundary movement, of any living modified organism resulting from modern
biotechnology that may have adverse effect on the conservation and sustainable use of
bioogical diversity, setting out for consideration, in particular, appropriate procedure
for advance informed agreement; . . .
AUTHOR’S NOTE: I would like to thank the Günter-Altner-Foundation
and the Hatzfeldt-Foundation for supporting the work for this contribution. My
participation in the Working Group on Biosafety was made possible by financial support by
the BUND (Friends of the Earth Germany), the Forum Environment and Development, and the
Günter-Altner-Foundation.
References
Abou-Donia, M. B. (1976). Physiological effects and metabolism of
gossypol. In F. A. Gunther & J. D. Gunther (Eds), Residue Reviews - Residues of
Pesticides and other Contaminants in the Totel Environment (Vol. 61, pp. 126-158). New
York: Springer-Verlag.
Anonymus. (no date). Characteristics of important commercial
varieties of Indian cottonseed. Technology Series 10. Hyderabad, IN: Indian Central
Oilseed Committee.
Berardi, L. C., & L. A. Goldblatt. (1980). Gossypol. In I. E. Liener
(Ed), Toxic Constituents of Plant Foodstuffs (pp. 183-237). New York: Academic
Press.
Carter, F. L., A. E. Castillo, V. L. Frampton, & T. Kerr. (1966). Phytochemistry,
5, 1103-1112.
Food and Drug Administration (FDA). (1992). Statement of
policy: Foods derived from new plant varieties (Docket No. 92N-0138). Federal Register,
57, 22984-23005.
Ismailov, A. (1959). Chemical Investigation of Gossypol, the Specific
Pigment of Cottonseed, in Russian. Tashkent, Uzbekistan: Author’s Summary.
Markman, A. L., & V. P. Rzhekhin. (1969). Gossypol in cotton plant.
Content and localization. In A. L. Markman & V. P. Rzhekhin (Eds), Gossypol and its
derivates (pp. 1-9). Jerusalem: Israel Program for Scientific Translation.
Murti, K. S., & K. T. Achaya. (1975). Cottonseed Chemistry and
Technology in its Setting in India. New Delhi: CSIR.
Murti, K. S., & B. Appu Rao. (1963). Studies on the compositional
and varietal characteristics of Indian cottonseed during the decenium 1951-52 to 1960-61.
Hyderabad, IN: Indian Central Oilseed Committee, 1963.
Narayana Rao, & M.; K. Krishnamurthy. (1953). Bull. cent. Fd.
tech. Res. Inst., 3,: 103.
Nida, D. L., S. Patzer, P. Harvey, R. Stipanovic, R. Wood, & R. L.
Fuchs. (1996). Glyphosate-tolerant cotton: The composition of the cottonseed is equivalent
to that of conventional cottonseed. Journal of Agricultural and Food Chemistry, 44,
1967-1974.
Smirnowa, M. I. (1936). Bulleten Prikladnoi Botaniki,
Series III, 15, 227.
Hartmut Meyer, from 1997 to the present, has been an observer of German
nongovernmetnal organizations (NGOs) at the negotiations of the Biosafety Protocol in
Montreal, Canada, and at the Conference of the Parties to the Convention of Biological
Diversity in Bratislava, Slovakia. His Ph.D. thesis was on the control of gene expression
by a circadian biological clock in tomatos, and his post-doctoral work was on microbial
and biochemical activities in forests soils in the Research Center of Forest Decline in
Göttingen. In 1997, he was project leader at BUND (Friends of the Earth Germany):
"Genetic engineering in our every day life - a contribution to sustainibility?"
Since 1998, he is the coordinator of the Working Group on Biological Diversity, German NGO
Forum on Environment and Development. Since 1999, he is coordinator of the European NGO
biotechnology network GENET.
Working Group on Biological Diversity, German NGO Forum
on Environment and Development, Reinhäuser Landstr. 51, D-37083 Göttingen, Germany;
phone: 49-551-7700027; fax: 49-551-7701672; email: hartmut.meyer@bund.net. |

